For movement disorders including Parkinson’s disease and Huntington’s disease
Digital biomarkers solution for clinical trial sponsors
Digital biomarkers can identify and measure clinically relevant signals in neurological movement disorders. Learn more about the solution for clinical trial sponsors from Roche.
For movement disorders including Parkinson’s disease and Huntington’s disease
Digital biomarkers solution for clinical trial sponsors
Digital biomarkers can identify and measure clinically relevant signals in neurological movement disorders. Learn more about the solution for clinical trial sponsors from Roche.
Why sensitive and objective measurements matter
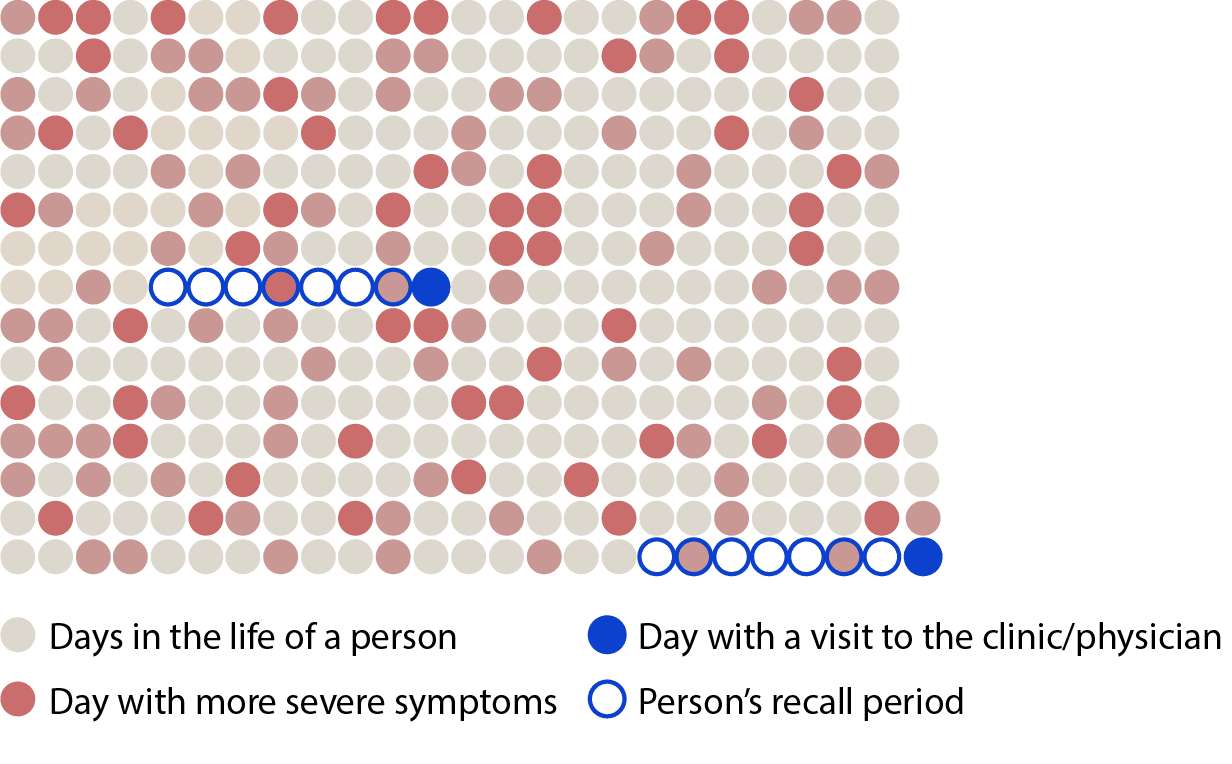
365 days in the life of a patient
People living with neurological conditions, such as Parkinson’s disease and Huntington’s disease, may not entirely recall the manifestation and severity of the disease symptoms during periodic in-clinic visits.
Occasional in-clinic assessments over the span of months are subject to poor inter and intra-rater reliability.
Benefits of remote at-home digital assessment solution for life science and clinical trial sponsors
Remote, at-home digital assessments offer continuous data collection and may yield more meaningful insights – and provide greater sensitivity – for clinical trial researchers.1-13
 Frequency
Frequency
 Resolution
Resolution
 Accuracy
Accuracy
 Reliability
Reliability
 Ecological validity
Ecological validity
Resources
Parkinson’s disease
Roche Digital Biomarker Solution demonstrates adherence, user satisfaction, and initial evidence of clinical validity
in clinial trial for Parkinsons disease
Parkinson’s disease
Digital Biomarkers Solution shows pathway to reduced clinical trial sample sizes, shorter durations
Parkinson’s disease (PD) clinical trial sponsors may gain an earlier indication…
Huntington’s Disease
Digital Biomarkers Solution may enable smaller and faster clinical trials
Huntington’s disease (HD) clinical trial sponsors may gain an earlier indication…
A remote assessment platform to support disease progression measurement for movement disorders
Remote assessment platform collects, stores and processes digital clinical data to support clinical trial research particularly for motor disorders and neurological conditions, such as Parkinson’s disease and Huntington’s disease:
Roche Digital Biomarker Assessment Suite
Subjects do active in-clinic tests under supervision, and active and passive monitoring as part of their at-home daily routine.
Digital Health Tool with clinical trial services
The solution is designed to support patient and site workflows in clinical trials, complete with technology, data collection, and analytics.
Pathway to the future
More than 14,000 months of Parkinson’s disease data with extensive use in clinical trials and more than 50 scientific publications, posters, and presentations since 2015.* Researchers and clinical trial sponsors now have access to the Roche Digital Biomarker Solution to accelerate their own research.
*View the Publication table
References & notes
- Pagano G. et al. N Engl J Med 2022;387:421-32. DOI: 10.1056/NEJMoa2202867(supplement appendix)
- Lipsmeier, F. et al. Reliability and validity of the Roche PD Mobile Application for remote monitoring of early Parkinson’s disease. Sci Rep 12, 12081 (2022).
- Lipsmeier, F. et al. Reliability and validity of the Roche PD Mobile Application for remote monitoring of early Parkinson’s disease. Sci Rep 12, 12081 (2022).
- Fehlmann et al. 2021, Relationship of satisfaction and adherence in remote digital monitoring: Results from a clinical drug trial in early Parkinson’s disease [abstract]. Mov Disord. 2021; 36 (suppl 1).
- Lipsmeier, F., et al. Digital health technology (DHT) derived features as sensitive measures of disease progression in early Parkinson’s disease under stable dopaminergic treatment Mov Disord. 2024; 39 (suppl 1).
- Fehlmann, B., et al. Patient perspectives on symptoms and functional impacts to inform a digital assessment of Parkinson’s disease. MDS Congress 2023.
- Fehlmann, B., et al. Patient perspectives on symptoms and functional impacts to inform a digital assessment of Parkinson’s disease. MDS Congress 2023.
- Kwasny, D., et al. Reliability and validity of automatedly generated vowel space metrics as biomarkers of articulatory impairment severity and progression in early Parkinson’s Disesase. MDS Congress 2023.
- Rukina, D., et al. Digital health technologies (DHT) can reduce sample size and enable shorter proof of concept clinical trial in Parkinson’s disease. ADPD 2023.
- Kwasny, D., et al. RELIABILITY AND VALIDITY OF DIGITAL SPEECH FEATURES AS BIOMARKERS OF DYSARTHRIA SEVERITY AND PROGRESSION IN INDIVIDUALS WITH EARLY PARKINSON’S DISEASE. ADPD 2023.
- Dorsey ER et. al. Novel methods and technologies for 21st-century clinical trials: a review. JAMA Neurol. 2015 May; 72(5):582-8.
- Goetz CG, et al. Movement disorder society UPDRS revision task force. movement disorder society-sponsored revision of the unied Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008 Nov 15;23(15):2129-70.
- Please see the publication table for a complete list of references.
Disclaimer
The Roche Digital Biomarker Solution for Parkinson’s disease is intended to collect, store and process digital clinical data to support exploratory research in clinical trials. This solution is not meant to be used for diagnosis and treatment decision making for individual patients.